How to Read a Cannabis Certificate of Analysis (COA)
AUTHORED BY: GENESTER WILSON-KING, MD FACOG & SARAH RUSSO
Overview
With almost anything we buy, there are certain parameters in place to ensure product safety and quality. CBD offerings are unique because people who use them cannot assume they are safe. The good news is that consumers can find high-quality CBD products if they do their research. This is why we are going to explain the importance of requesting and reviewing a Certificate of Analysis (COA), which should be available for any cannabis product that you use.
A Certificate of Analysis is a document from an accredited laboratory that confirms that a regulated product meets certain specifications. A COA commonly contains the testing results performed as part of the quality control process. Most states with cannabis programs require laboratory testing on all products. However, CBD products lack testing in general. Many CBD products do not fall under state guidelines.
If you are using botanical preparations for optimal health, then a product should provide therapeutic benefits without safety concerns. That’s why product testing is of the utmost importance. Many CBD companies choose to have their products tested independently. In this instance, the company decides what tests should be done. It is the same situation as your physician ordering blood tests for you. The lab performs the tests that have been requested.
Cannabis testing can be performed on a random sample of the raw, harvested crop before and after processing. The final product should then be tested by an accredited third party laboratory. It is crucial to know what you are taking. The test results should be available on the company’s website. If they are not, contact the company and request it. If they are unresponsive or report that the information isn’t available, then don’t use their product. Several studies [1,2,3] have shown that more than 70% of the CBD products sold online are mislabeled. Some of them did not have CBD in them at all. [4]
Machines used to test cannabis include gas chromatography (GC) and high performance liquid chromatography (HPLC). Both of these methods are commonly used in analytical chemistry to determine the purity of a substance or the concentration of components. There are differences between these two methods but both provide extensive insight on the components of botanical (and other) products.
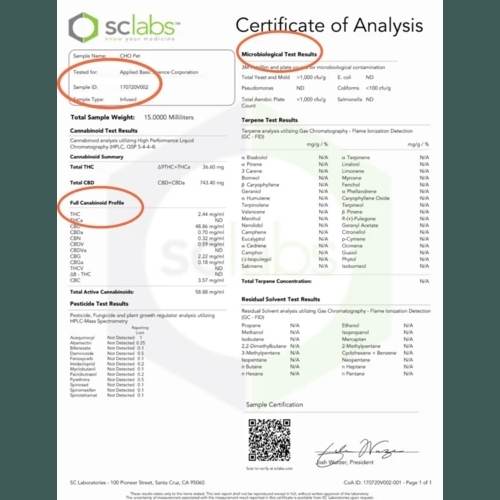
The Certificate of Analysis will look similar to the sample report below. There are many tests that can be ordered. A CBD product can be analysed for cannabinoids, terpenes, and the presence of microbiological, pesticides, and residual solvents. Follow along as we walk you through the sample COA!
How to look at the analysis
At the top of the COA, you will find the product name, the company who ordered the testing, batch number, and the date. The date and batch number should match the product that is under consideration for purchase. If the date is old or there is a different batch number, that may be a sign the company does not do enough testing or is behind on updating their information. Contact the company for the COA that matches the batch number of your product. A caveat is if the product is “white labeled” for the company. White-labelling is a product produced by one company that another entity rebrands to make it appear as if they had made it. The COA will show the company that requested the test. Questions about the product should be addressed to them.
The body of the report will generally have the test results grouped into categories. Most COAs will report the results in milligrams per gram. In the case of tinctures, 1ml of tincture will weigh about 1 gram. If there is a LOQ (%) column, that means a limit of quantitation which is the smallest amount detectable by the laboratory’s equipment.
Cannabinoid Concentration
The cannabinoid profile is a listing of all the cannabinoids found in the product. That includes the major and minor cannabinoids, such as THC, CBD, CBC, CBG. If it is a CBD hemp-based product, the amount of THC should be 0.3% or below. (Learn more in Hemp vs. Marijuana). Synthetic cannabinoids will also show up here (read below for further info on them).
The cannabinoid and terpene test profiles are often called “Potency Test Profiles”.
Terpene Profile
Terpenes are a group of substances that give plants their various scents. Terpenes are an evolutionary tool to attract pollinators and deter predators. Cannabis has unique terpenes and distinct aromas as well. Terpenes and cannabinoids come from the same glands on the cannabis plant (the trichomes). Terpenes have aromas while cannabinoids have no scent. Terpenes are also responsible for some of the various therapeutic properties of cannabis. [5] Terpenes will be discussed in detail in another blog post.
Flavonoid Test Profile
There are 23 flavonoids identified in cannabis. Flavonoids are nutrient-rich pigments found in many plants, vegetables, fruits, and trees. They give botanicals their color. Flavonoids can trigger biochemical reactions in the body. These compounds may also contribute to the therapeutic effects of different cannabis varieties. Cannaflavin A, B, and C are examples of unique flavonoids found in cannabis. [6]
Genetic Testing
The genetic characteristics of different cannabis varieties (mistakenly referred to as “strains”) are of interest for both researchers and consumers. The physical and genetic characteristics of the plant is important for cultivators trying to grow specific types of cannabis for various purposes. For example, studies in Israel are looking at various genotypes and phenotypes of cannabis to target specific cancers. [7]
Microbiological Testing
To truly be considered safe, your CBD product should have microbiological testing. These analyses ensure that your product is not contaminated with harmful microorganisms such as salmonella, mold, yeast, or other biological contaminants that you wouldn’t want in your body.
Synthetic Cannabinoids
Synthetic cannabinoids are human-made chemicals designed to produce a psychoactive effect similar to THC. Sometimes they are sprayed on the raw cannabis plant or mixed with vape pen oil or other products. They are often put in CBD products to achieve a “high”. Common names are “K2” and “Spice”.
Synthetic cannabinoids are often more potent than naturally occurring THC. The human body is not equipped with the tools needed to break them down. While THC is a partial agonist to the CB1 receptor in the brain, synthetic cannabinoids are full agonists. These compounds tightly bind to CB1 receptors and are hard for the body to remove. That’s why there are potentially dangerous side effects.8 (Refer to the What is the ECS? blog for more information on cannabinoid receptors).
To ensure a safe CBD product, make sure the ingredients contain only naturally occurring cannabinoids and plant components. Synthetic cannabinoids, if present, would show up within the cannabinoid testing panel.
Residual Solvents
Your product should also be tested for residual solvents. Solvents are sometimes used to extract the medicinal compounds of a CBD product. Each solvent will extract different compounds from the plant and have their pros and cons. Examples of solvents include ethanol or butane. These solvents can leave residues which must be cleaned out from the final product. The solvent tests will detect any leftover residues.
Carbon Dioxide (CO2) is a technique that allows for a clean extraction of cannabis products. CO2 extraction should be done by experts. Some solvents, such as acetone, benzene, and propane should never be used to extract a CBD product. [9] No matter what type of extraction method used, there should be no evidence of solvent residues showing up in the final product.
Pesticides
Pesticide use in cannabis products is very common and a serious concern. A 2016 study found that 84.6% of cannabis products available in the legal market in Washington state tested positive for pesticide residues. If pesticides are used in cannabis production, they are concentrated into the final product. The presence of dangerous pesticides have documented health risks. These compounds come in the form of toxins that hinder the process of the neurological, developmental, hormonal, and reproductive systems within the body. Many of these pesticides are harmful even if present in very small amounts. A CBD product should not be cultivated using any pesticides or fungicides that is not on the approved list of agents. Both the American Herbal Products Association and American Herbal Pharmacopoeia have developed medical cannabis guidelines for cultivation and production. [10] Pesticide residue should not be present in any product you are going to take.
Mycotoxins
Mycotoxins are specific species of mold that can contaminate CBD products. There are several types of mycotoxins which are categorized into groups. Aflatoxins are probably the most common and the most harmful. Other major types of mycotoxins include citrinin, ergot, fusarium, ochratoxin, and patulin. [11]
Heavy Metals
Cannabis is a bioaccumulator that cleans the soil it is grown in. If there are heavy metals present in the plant afterwards, it should not be used for human consumption (More information: Marijuana vs. Hemp). Testing for heavy metals in CBD products is crucial. Remnants of antimony, arsenic, copper, nickel, lead, selenium, silver, mercury, zinc, and others can be toxic if ingested in certain amounts. You don’t want to see any heavy metals showing up on a laboratory analysis!
Water Activity and Moisture Content
You may also see the water activity and moisture content categories on a COA. The moisture content is a measurement of the total amount of water contained in the product. The water activity measures the “excess” amount of water that’s available for microorganisms to use. It is important that manufacturers understand the optimal water activity needed to control the growth of pathogens and prevent spoilage.
If your product has a water activity range above .85, it will have to be refrigerated or use another technique to control the growth of pathogens. If the product has a water activity range between .60 and .85, it won’t require refrigeration. But it will have a limited shelf-life due to its susceptibility to grow yeasts and molds. If a product has a water activity below .60, it will be shelf stable even without refrigeration. [12]
These two tests are often not performed on cannabis products, but you may see the categories on a COA. We want you to know about it in case you see it.
Further Considerations
The end of the report will have the name, date, and signature of the technicians who analyzed the sample. This establishes the authenticity of the COA. There should also be the lab’s name, contact information, and certification number. Make sure that the lab issuing the report is different from the company selling the products. This assures the report is from a third party and not from the company that makes the product.
On the sample COA below, there isn’t a section for flavonoid profiles, heavy metals, mycotoxins or genetic tests. That is because they were not ordered. This isn’t good or bad, unless you are looking for those specific tests. You can always contact the company and ask questions.
The Product Label
Remember that CBD products are mislabeled more often than not. Beware of products that make claims to cure cancer, autism, etc. The FDA does not support any medical claims for CBD except for Epidiolex as indicated in the treatment of specific seizure disorders (Lennox-Gastaut syndrome and Dravet syndrome). The state of Florida also requests its CBD approved licensees abide by these rules. Reputable companies will adhere to these guidelines. [13]
Does the amount of CBD on the bottle match what was shown on the COA (give or take 5mg)? Are there any other ingredients? MCT (medium chain triglyceride) is fractionated coconut oil often used to make cannabis products easier to apply. It is generally well tolerated. Companies often add other herbs or ingestible essential oils to make the product more palatable. Sometimes additional ingredients are to boost the therapeutic impact of the CBD oil. Make sure you are not allergic to any of them.
Summary
There are a multitude of CBD (cannabidiol) products on the market. Cannabidiol products can be purchased in many places such as gas stations, grocery stores, pharmacies, some physician’s offices, and online. The regulations for these products are different depending on where you live. Consumers can find high-quality CBD products if they do their research. Hopefully, this blog post has helped to provide some guidance.
Sample COA

 Genester Wilson-King, MD FACOG is a Board-Certified Obstetrician and gynecologist with over 25 years of clinical experience providing compassionate and research-driven care to patients. After years of working as a full-service OB/GYN, she founded Victory Rejuvenation Center (VRC), a private holistic and preventive medicine practice that provides life-transforming management modalities and customized medicines to patients. She is the Medical Advisor to Treadwell Farms.
Genester Wilson-King, MD FACOG is a Board-Certified Obstetrician and gynecologist with over 25 years of clinical experience providing compassionate and research-driven care to patients. After years of working as a full-service OB/GYN, she founded Victory Rejuvenation Center (VRC), a private holistic and preventive medicine practice that provides life-transforming management modalities and customized medicines to patients. She is the Medical Advisor to Treadwell Farms.
As the Medical Director of VRC, Dr. Wilson-King provides services that help her patients age gracefully and achieve holistic well-being. She focuses on plant-based medicine, integrated health, nutrition, supplements, cannabis education, and hormone balance.
Dr. Wilson-King is Co-Vice President of the Society of Cannabis Clinicians (SCC). The SCC is an educational and scientific society of physicians and other professionals dedicated to the promotion, protection and support of cannabis for medical use. Dr. Wilson-King co-authored the Best Practices Guidelines for Cannabis Use in Pregnancy and Breastfeeding, and Cannabis Use in Women – Special Considerations (in progress). She is also on the Board of the Doctors For Cannabis Regulation (DFCR), the first and only national physicians’ association dedicated to the legalization and regulation of cannabis for adults. Advancing the DFCR’s commitment to addressing the disproportionate criminalization of cannabis use among communities of color and the nation’s poor, she regularly provides expert opinions for legal cases involving cannabis.
Dr. Wilson-King is a nationally recognized advocate, clinician, and educator for cannabis and hormone and wellness therapies. She presents on cannabis use in obstetrics and gynecology, hormone therapy for PMS, various stages of menopause, and for applications in nutrition.
 Sarah Russo is a longtime plant enthusiast and globetrotter. She got her degree in environmental studies and social justice, with a focus on plant medicine from the Evergreen State College. She is a freelance writer, consultant, and project manager with over 13 years of experience in the cannabis and herbal medicine space. Her main objectives are fighting for the right to use plants, implementing social justice approaches in the cannabis industry, as well as encouraging sustainable agricultural practices. She is currently based on an island in the Mediterranean. Sarah is a content creator for Treadwell Farms.
Sarah Russo is a longtime plant enthusiast and globetrotter. She got her degree in environmental studies and social justice, with a focus on plant medicine from the Evergreen State College. She is a freelance writer, consultant, and project manager with over 13 years of experience in the cannabis and herbal medicine space. Her main objectives are fighting for the right to use plants, implementing social justice approaches in the cannabis industry, as well as encouraging sustainable agricultural practices. She is currently based on an island in the Mediterranean. Sarah is a content creator for Treadwell Farms.
Sources
- Bonn-Miller, M. O., Loflin, M. J., Thomas, B. F., Marcu, J. P., Hyke, T., & Vandrey, R. (2017). Labeling accuracy of cannabidiol extracts sold online. Jama, 318(17), 1708-1709.
- Penn Medicine. (2017). "Penn Study Shows Nearly 70 Percent of Cannabidiol Extracts Sold Online Are Mislabeled". Penn Medicine News
- Rubin, R. (2019). Cannabidiol Products Are Everywhere, But Should People Be Using Them? JAMA. 322 (22), 2156–2158.
- Hansen, Claire. (2019). "1 in 7 Adults Use CBD Products, Gallup Survey Finds". US News.
- Rahn, Bailey. (2014). "What are cannabis terpenes and what do they do?" Leafly. Accessed on 5/19/2020.
- Frye, Patricia. The Medical Marijuana Guide: Cannabis and Your Health. Roman and Littlefield Publishing, 2018.
- Baram, L., Peled, E., Berman, P., Yellin, B., Besser, E., Benami, M., ... & Meiri, D. (2019). The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget, 10(41), 4091.
- Cushing, D., Goakar, D., Joseph, B., & ImmunAG, L. L. P. Synthetic cannabinoids severely elevate amino transferase levels. Natural cannabidiol does not. Journal of Medical Phyto Research ISSN, 2(1), 1-13.
- Tatera, Kelly. (2017). "Residual Solvent Analysis: Ensuring the safety of cannabis extracts". Analytical Cannabis. Accessed on 5/19/2020.
- Russo, E. B. (2016). Current therapeutic cannabis controversies and clinical trial design issues. Frontiers in pharmacology, 7, 309.
- Modern Canna. (2020). Florida Cannabis Testing. Accessed on 5/19/2020.
- Bogart, John. (2018). Moisture Content vs Water Activity: Use Both to Optimize Food Safety and Quality. Kett: Science of Sensing. Accessed on 5/19/2020.
- Lazarus Naturals. (2019). How to Identify Fake CBD. Accessed on 5/19/2020.






